Generally, most of the methods for detecting COD in water have strict requirements on the content of chloride ions. Because chloride ion is currently the biggest interference factor in COD water quality testing, although there are some treatment methods in existing methods, there are still certain limitations in actual testing. And today we are with you to understand a method to detect COD at high chloride ion content.
The principle of this method is to add a suitable concentration of sulfuric acid to the high chloride ion water sample, and under the conditions of heating and blowing, the chloride ions are released in the form of hydrogen chloride, so as to achieve the purpose of removing the interference of chloride ions. Concentrated volumes of sodium hydroxide are absorbed to avoid efflux to the environment. After removing the chloride ion, add a known amount of potassium bicarbonate solution to the water sample, and use silver salt as a catalyst in a strong acid medium. Titrate the unreduced potassium bicarbonate in the water sample, and convert the consumed amount of ferrous sulfate into the mass concentration of consumed oxygen.

1. Sulfuric acid.
2. Silver sulfate.
3. Sulfuric acid solution I: 4+1 (v/v).
4. Sulfuric acid solution II: 1+9 (v/v).
5. Sodium hydroxide solution: 0.05mol/L.
Weigh 2.0 g of analytically pure sodium hydroxide in a beaker, dissolve with water and dilute to 1000 mL.
6. Mercury sulfate solution: 100g/L.
Weigh 10 g of analytically pure mercury sulfate, dissolve it in 100 mL of sulfuric acid solution II, and mix well.
7. Standard stock solution of potassium dicarboxylate: 0.250mol/L.
Weigh 12.258g of the benchmark reagent potassium dicarboxylate dried to constant weight in an electric oven at 120℃±2℃, dissolve in water, transfer it into a 1000mL volumetric flask, dilute with water to the mark, and shake well. Stored in a refrigerator at 4°C, the validity period is 6 months. Commercially available standard solutions are also available.
8. Standard solution of potassium dicarboxylate: 0.0250mol/L.
Pipette 50.00mL of potassium dicarboxylate standard stock solution into a 500mL volumetric flask, dilute to the mark with water, and shake well. Stored in a refrigerator at 4°C, the validity period is 2 months.
9. Standard stock solution of ferrous sulfate; 0.05mol/L.
Weigh 19.5g of analytically pure ferrous sulfate, dissolve it in water, add 10mL of sulfuric acid, transfer the solution to a 1000mL volumetric flask after cooling, dilute to the mark with water, and shake well. Stored in a refrigerator at 4°C, the validity period is 6 months.
10. Standard solution for ferrous sulfate: 0.005mol/L.
Pipette 50.00mL of standard stock solution of ferrous sulfate into a 500mL volumetric flask, dilute to the mark with water, and shake well. Stored in a refrigerator at 4°C, the validity period is 2 months.
11. Before use every day, the concentration of the standard solution of ferrous ferrous sulfate must be accurately calibrated with the standard solution of potassium dicarboxylate.
12. Take 5.00mL potassium bicarboxylate standard solution and put it in a conical flask, dilute it with water to about 50mL, slowly add 15mL sulfuric acid, mix well, add 3 drops (about 0.15mL) of test ferrous indicator after cooling, Using ferrous sulfate standard titration, the color of the solution changes from yellow to blue-green to red-brown as the end point.
13. Test the ferrite indicator solution. Weigh 0.7g of analytically pure ferrous sulfate heptahydrate, dissolve it in 50mL of water, add 1.5g of analytically pure 1,10-phenanthrene, stir until dissolved, dilute to 100mL, and store in a brown bottle.
14. Potassium hydrogen phthalate standard solution: 2.0824mmol/L.
Weigh 0.4251g of the benchmark reagent potassium hydrogen phthalate dried in an electric oven at 105℃±2℃ for 2 hours, dissolve in water, transfer it into a 1000mL volumetric flask, dilute with water to the mark, and shake well. Stored in a refrigerator at 4°C, the validity period is 2 months. Commercially available standard solutions are also available. Taking potassium bicarbonate as the oxidant, the CODcr value of the complete oxidation of potassium hydrogen phthalate is 1.176g oxygen per gram (that is, 1g potassium hydrogen phthalate consumes 1.176g of oxygen), so the theoretical CODcr value of this standard solution is 500mg/L.
1. Explosion-proof glass beads or zeolite.
2. Constant temperature water bath acidification blowing device
3. CODcr reflux device: an all-glass reflux device with a 250mL ground-mouth CODCr digestion bottle with a grinding mouth size of Φ=40mm and a height of 18cm (or 21cm) can be used. Other specifications of CODcr digestion bottles can also be used, which need to be verified before use. It is recommended to use a water-cooled or air-cooled all-glass recirculation device, and other equivalent condensing recirculation devices are also acceptable.
4. CODcr heating device: the heating device matching the CODcr reflux device.
5. Balance: Sensitive amounts are 0.0001g and 0.01g.
6. Acid burette: 25mL or 50mL.
7. Electric oven.
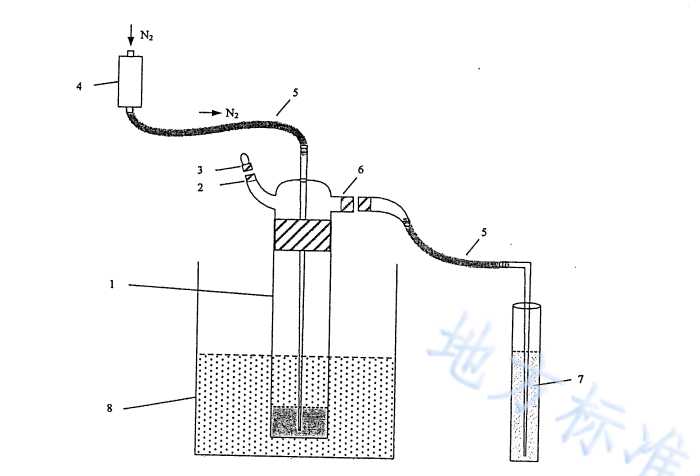
Take 10.0 mL of the fully shaken water sample in a CODcr digestion bottle, move it to a constant temperature water bath acidification blower that has been preheated to 70℃±2℃, fix the digestion bottle to keep it stable, and connect the acid blower according to the instructions device, add 50 mL of sodium hydroxide solution to the hydrogen chloride absorption tube. Add 20 mL of sulfuric acid solution I from the acid addition port while it is still hot, turn on nitrogen, and after checking the air tightness of the device, blow air at a speed of 400 mL/min for 50 min.
After acidizing and blowing, turn off the nitrogen gas, remove the connecting tube, take out the CODcr digestion bottle, and add 2 mL of mercury sulfate solution, 5.0 mL of potassium bicarboxylate standard solution, 0.15 g of silver sulfate, explosion-proof boiling point to the sample after the chloride ion has been removed. Glass beads or zeolite, shake well.
Connect the CODcr digestion bottle to the lower end of the condenser tube of the reflux device, and continuously rotate the CODcr digestion bottle to mix it evenly. Since the solution started to boil, the reflux was kept at a slight boil for 2h.
After refluxing and cooling, 45 mL of water was added from the upper end of the condenser to rinse the condenser, and the CODcr digestion bottle was removed.
After the solution was cooled to room temperature, add 3 drops of ferrous ion indicator solution, titrate with standard ferrous sulfate, and the color of the solution changes from yellow to blue-green to red-brown as the end point. Note down the consumption volume V1 of the standard solution of ferrous sulfate.
Samples with CODcr concentration >50mg/L can be diluted and measured.
Then follow the same steps to replace the tested water sample with 10.0mL laboratory first-grade pure water to carry out the blank test, and record the volume V0 of the standard solution of ferrous ammonium sulfate consumed during the blank titration.
Finally, the concentration of chemical oxygen demand is calculated according to the relevant formula.
最新动态
相关推荐