Reclaimed water generally refers to the water body whose water quality index reaches the corresponding use standard stipulated by the state after a series of purification treatment of wastewater or other sewage. At present, domestic reclaimed water is mainly used for industrial use, urban landscape water, and river ecological water replenishment. aspect. The use of reclaimed water is a very beneficial measure for some water-scarce cities. But at the same time, we also need to do a good job in the water quality testing of reclaimed water. Today, we will share with you the steps to detect the mass concentration of chromium in reclaimed water by voltammetry.
Voltammetry adopts the principle of non-stoichiometric method, allowing hexavalent chromium in the water sample to react with diethylenetriaminepentaacetic acid (DTPA), and the resulting product is absorbed on the suspended mercury electrode within a certain current and voltage range. Adsorption enrichment. When the electrode potential is uniformly scanned in the positive and negative directions, and the potential reaches the level where the enriched complex reacts, the metal complex enriched on the electrode forms ions into the solution. The wave potential is used for qualitative analysis, the peak height is used for quantitative analysis, and the content of hexavalent chromium ions to be measured is calculated by the standard addition method. When measuring total chromium, all chromium elements are first oxidized to hexavalent chromium in the presence of strong oxidizing states, and then measured. The trivalent chromium content was obtained by subtracting the total and hexavalent chromium results. When the organic matter in the sewage seriously interferes with the determination of chromium, this method is only applicable to the determination of total chromium.

1. The reagents used in this standard, unless otherwise specified, should use high-grade pure reagents and meet the requirements of first-grade water in GB/T6682.
2. The standard solution of impurities required in the test shall be prepared in accordance with the provisions of GB/T602 unless other requirements are specified.
3. Choose a non-ferrous spoon when weighing solid reagents.
4. Nitric acid solution: 1 x 1.
5. Nitric acid solution: 1+50.
6. Sulfuric acid solution: 1 ten 120.
7. Sodium hydroxide solution: 500g/L.
8. Ammonium persulfate solution: 1g/L. The solution is ready to use
9. Electrolyte: Weigh 1.64g of anhydrous sodium acetate, 1.96g of diethylenetriaminepentaacetic acid (DTPA) and 21.3g of sodium nitrate. After dissolving in water, adjust the pH to 6.2-6.3 with sodium hydroxide solution and dilute to 100ml.
10. Chromium standard stock solution: 0.1 mg/mL.
11. Chromium standard solution: 2504g/L. Accurately pipette 250uL of chromium standard stock solution into a 100mL plastic volumetric flask, and dilute to the mark with water. The solution is used now.
12. Mercury: purity ≥99.999%.
13. Nitrogen: purity ≥99.999%.
1. Voltammetry.
2. Working electrode: Suspended mercury electrode, filled with an appropriate amount of mercury before use, and sealed.
3. Reference electrode: silver/silver chloride reference electrode.
4. Shop auxiliary electrode: platinum auxiliary electrode.
5. Pipette: 100uL, 1000uL.
1. All utensils are soaked in nitric acid solution (1+1) for more than 12 hours before use, and then rinsed with water.
2. The electrolytic cell is rinsed with nitric acid solution (1+50) before the water sample measurement, and then rinsed with water, and the electrode system is also treated in the same way.
3. Connect the voltammetric polarograph and the dosing device, and carry out the electrode test and standard solution test according to the requirements of the instrument and electrode instruction manual to ensure the stability of the instrument.
Accurately pipette 1000uL water sample (V,) into the electrolytic cell, accurately add 2.50mL electrolyte and 6.50mL water, adjust the pH of the solution to 6.2±0.1 with sodium hydroxide solution or glacial acetic acid, the total volume at this time is V, . Insert the electrode, start stirring and deoxygenate with nitrogen (partial pressure 0.15MPa) for 5min. Close the nitrogen valve, keep stirring, and set according to the relevant instrument test conditions for pre-electrolysis enrichment. Stop stirring for 10s, carry out the dissolution scanning measurement according to the set scanning potential range and scanning rate, and record the dissolution voltammetry curve and the peak current value I0 of hexavalent chromium. After that, referring to the peak current value of the sample, add a certain volume (Vs, 10ul-200uL) of chromium standard solution, measure the peak current value I1 after adding the standard, and then add the same volume (Vs) of chromium standard solution, and measure the addition of the standard solution again. The marked peak current value I2. The volume of the added chromium standard solution should be appropriate, and the increment of the peak current value after adding the standard solution should be as close to the initial peak current value of the sample as possible. The concentration of the standard solution can be changed if necessary.
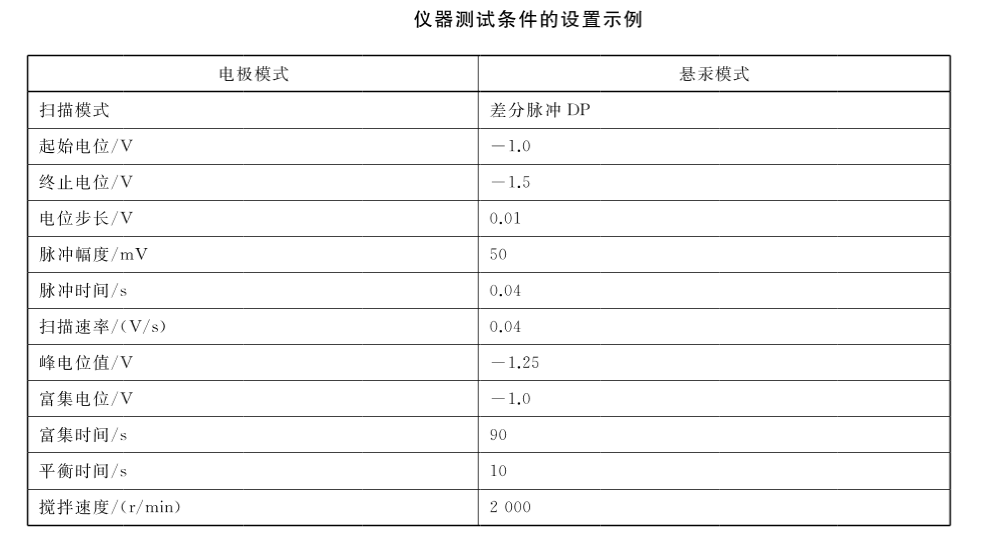
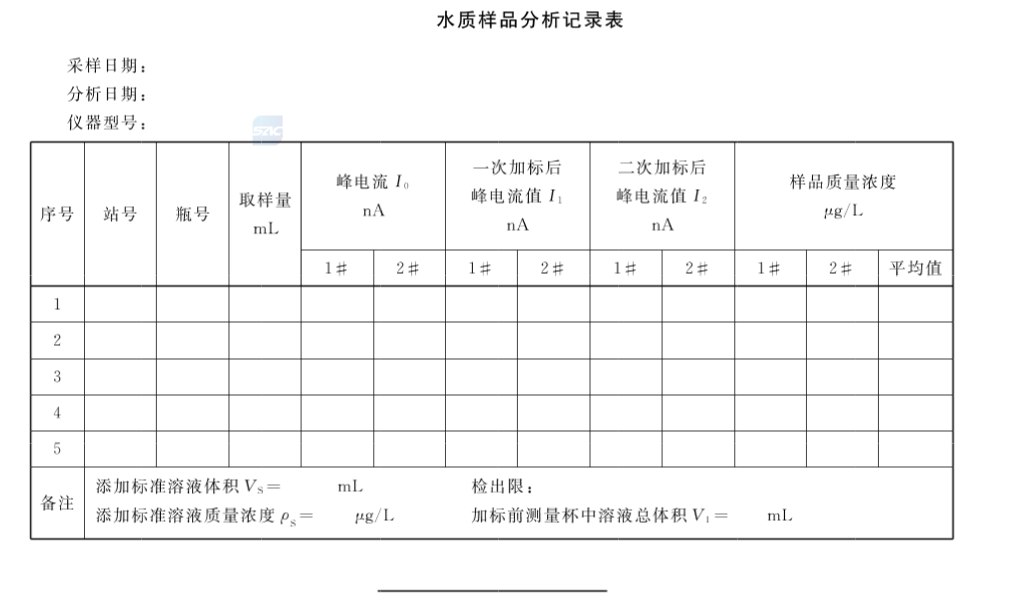
Taking the concentration of the added chromium standard solution (ug/L) as the abscissa, and the corresponding corrected peak current value (A) as the ordinate, draw the working curve of the standard addition method, and the intersection of the extrapolation and the abscissa is is the content of hexavalent chromium in the water sample. For sample analysis, please refer to the water quality sample analysis record sheet.
Pipette 100mL of the water sample to be tested in a conical flask, add 1mL of sulfuric acid solution and 10mL of ammonium persulfate solution, heat it to boiling on an adjustable electric furnace, keep it at a slight boil for about 35min, reduce the volume to about 50mL, and remove it from the water. Cool at room temperature. Transfer all to a 100ml. volumetric flask and dilute to volume with water. Then measure according to the operation steps of hexavalent chromium, that is, the content of total chromium in the water sample.
If the instrument has its own algorithm software, it can directly display the final detection result. If not, you can calculate it according to the relevant formula.
The above content comes from 《GB/T 37905-2019 Quality of reclaimed water-Determination of chromium-Voltammetry》
相关推荐