Method for the detection of methanol and acetone in water by gas chromatography
发布时间:2022/7/29 10:11:31 来源:贯奥仪器仪表 作者:便携式多参数水质分析仪器 阅读次数:
The principle of detecting methanol and acetone in water by headspace gas chromatography is that under certain temperature conditions, the volatile components in the sample in the headspace bottle volatilize to the headspace to generate vapor pressure, and after the gas-liquid two phases reach thermodynamic dynamic equilibrium , the volatile organic compounds in the gas phase were separated by gas chromatography and detected by a hydrogen flame ionization detector. Qualitative by chromatographic retention time, quantified by external standard method.

Methods of Interference and Elimination During Detection
When the chromatographic column of polyethylene glycol stationary phase is used for separation, when the concentration of ethyl acetate in the sample is higher than 25 mg/L, it will interfere with methanol, and methyl acetate will interfere with acetone. The stationary phase can be changed to 6% cyanide. Propylbenzene + 94% dimethylsiloxane column separation, if necessary, use gas chromatography-mass spectrometry for qualitative confirmation.
Reagents used for testing
1. Detection of water
Double distilled water or water prepared by pure water equipment. The concentration of the target compound in the experimental water must be lower than the detection limit of the method, otherwise, pretreatment should be performed. The interference of methanol and acetone in the experimental water can be removed by heating and boiling for 15 minutes, then capping and cooling, or by purging with an inert gas.
2. Hydrochloric acid 1.19g/ml.
3. Hydrochloric acid solution: 1+1.
Measure 100ml of hydrochloric acid, add it to 100ml of testing water and mix well.
4. Sodium chloride (NaCl): excellent grade pure.
Heating at 400°C for 2h to remove organic matter that may be adsorbed on the surface, and then store in a clean reagent bottle after cooling.
5. Ascorbic acid.
6. Methanol: pesticide residue grade.
7. Acetone: pesticide residue grade.
8. Methanol and acetone standard solution I: 3×104 mg/L, 3×103 mg/L.
Pipette an appropriate amount of experimental water into a 100ml volumetric flask and place it on a balance to weigh. Carefully add a few drops of methanol until the weight gain is about 3.0g (accurate to 0.1mg), weigh again, and calculate the exact mass of methanol added according to the difference between the two weighings. Continue to carefully drop a few drops of acetone until the weight gain is about 0.3g (accurate to 0.1mg), weigh again, and calculate the exact added mass of acetone based on the difference between the last two weighings. Dilute to the mark with experimental water, shake well, and calculate the exact concentration of methanol and acetone standard solution I (accurate to 1 mg/L). Ready to use.
9. Methanol and acetone standard solution II: 300mg/L, 30mg/L.
Accurately pipette 1.00ml of methanol and acetone standard solution I into a 100ml volumetric flask, dilute to volume with experimental water, and shake well.
10. Nitrogen: purity ≥99.999%.
11. Hydrogen: purity ≥99.999%.
12. Air: Oil-free compressed air, purified by 5Å molecular sieve.
Testing equipment
1. Gas chromatograph: with hydrogen flame ionization detector (FID), with split/splitless injection port, and the oven temperature can be programmed.
2. Automatic headspace sampler: The heating temperature range is controlled between room temperature and 250°C, and the temperature control accuracy is ±1°C.
3. Chromatographic column I: Quartz capillary column, 30m (length) × 530m (inner diameter) × 1.0m (film thickness), the stationary phase is polyethylene glycol, or other equivalent capillary columns.
4. Column II: Quartz capillary column, 30m (length) × 530m (inner diameter) × 3.0m (film thickness), the stationary phase is 6% cyanopropylbenzene + 94% dimethylsiloxane, or others efficient capillary column.
5. Analytical balance: the inductive amount is 0.0001g.
6. Headspace bottle: 22ml glass headspace bottle, with sealing gasket (PTFE/silicone rubber or PTFE/butyl rubber material), sealing cap (screw cap or disposable gland), can also be used Glass headspace vials for use with automatic headspace samplers.
7. Micro syringe: 5ul, 10ul, 25ul, 100ul, 250ul, 1.0ml injection needle.
8. Sampling bottle: 40ml brown glass bottle with a silicone rubber-PTFE lined screw cap.
9. General laboratory instruments and equipment.
Water sample collection and preservation
When collecting water samples, it is not allowed to wash with water samples, the water samples should overflow in the sampling bottle without leaving space, and the exposure of water samples to the air should be avoided or reduced as much as possible during sampling. All water samples were collected in parallel. If the water sample contains residual chlorine, 25mg (accurate to 0.001g) ascorbic acid should be added to the sampling bottle before sampling. If the residual chlorine content in the water sample exceeds 5mg/L, the amount of ascorbic acid should be increased proportionally. For every 5mg/L increase in the residual chlorine content, an additional 25mg (accurate to 0.001g) ascorbic acid should be added.
At the same time, put laboratory water in airtight glass bottles and bring it to the sampling site. Follow the above steps to collect full-program blank water samples. Each batch of water samples should bring a full-program blank.
water sample preservation
After the water sample is collected, an appropriate amount of hydrochloric acid solution should be added immediately to make the pH of the water sample less than or equal to 2, the bottle stopper should be tightened, the label should be affixed, and it should be immediately placed in a refrigerator for transportation at a temperature below 4°C. After the water samples are transported back to the laboratory, they should be refrigerated below 4°C, protected from light and sealed, and the analysis and determination should be completed within 14 days. The water sample storage area should be free of volatile organic compounds.
Sample preparation
After returning the water sample to room temperature, accurately pipette 10.0 ml of the water sample into the headspace bottle pre-added with 3.0 g of sodium chloride, seal it immediately, and shake well for testing.
When the actual water sample concentration exceeds the working curve range, the sampling volume of the water sample can be appropriately reduced and the volume is adjusted to 10.0ml before measurement.
For blank sample preparation, use experimental water instead of water sample, and prepare laboratory blank samples according to the same operation steps as sample preparation.
Detection steps
Headspace Injection Reference Conditions
Heating equilibrium temperature: 80°C; heating equilibrium time: 30min; sampling needle temperature: 100°C; transfer line temperature: 110°C; injection volume: 1.0ml.
Gas Chromatography Reference Analysis Conditions
Program temperature: the initial column temperature is kept at 50°C for 6 minutes, raised to 100°C at 5°C/min for 2 minutes, and then raised to 200°C at 5°C/min and held for 5 minutes; inlet temperature: 200°C; split ratio is 3:1 ; Carrier gas: nitrogen, flow rate: 5.0ml/min; detector temperature: 280°C; gas: hydrogen, flow rate 30ml/min; auxiliary gas: air, flow rate 300ml/min.
Establishment of working curve
Take 7 headspace vials, add 3.0g sodium chloride respectively, accurately pipette 10.00ml of experimental water to each headspace vial, and then add 0ul, 25ul, 50ul, 100ul standard use solution II) and 5ul, 10ul, 15ul standard solution respectively. Using liquid I, the standard series concentrations of methanol are respectively 0mg/L, 0.75mg/L, 1.5mg/L, 3.0mg/L, 15.0mg/L, 30.0mg/L and 45.0mg/L, the standard of acetone The serial concentrations are 0mg/L, 0.075mg/L, 0.15mg/L, 0.30mg/L, 1.50mg/L, 3.00mg/L and 4.50mg/L (this is the reference concentration), cover and shake well. According to the reference conditions of the instrument, the determination is performed sequentially from low concentration to high concentration. Take the mass concentration (mg/L) of the target substance as the abscissa and the peak area or peak height as the ordinate to establish a working curve.
Sample determination
The measurement of the sample is carried out under the same conditions as the establishment of the working curve. Alternating high-concentration samples with low-concentration samples may cause interference. When a high-concentration sample is analyzed, a blank sample should be analyzed to check for cross-contamination.
Target compound characterization The target compound can be quantified according to the component retention time. Under the chromatographic conditions referenced in this standard, the standard chromatogram of methanol and acetone is shown in Figure 1. When there are interfering peaks, chromatographic column II can be used as an auxiliary qualitative method. The standard chromatogram of methanol and acetone in chromatographic column II is shown in Figure 2. When necessary, confirmation of qualitative results should be carried out by gas chromatography-mass spectrometry.
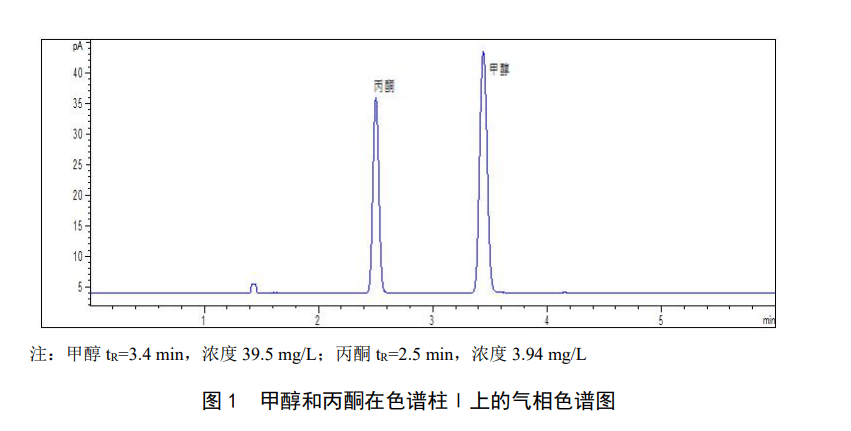
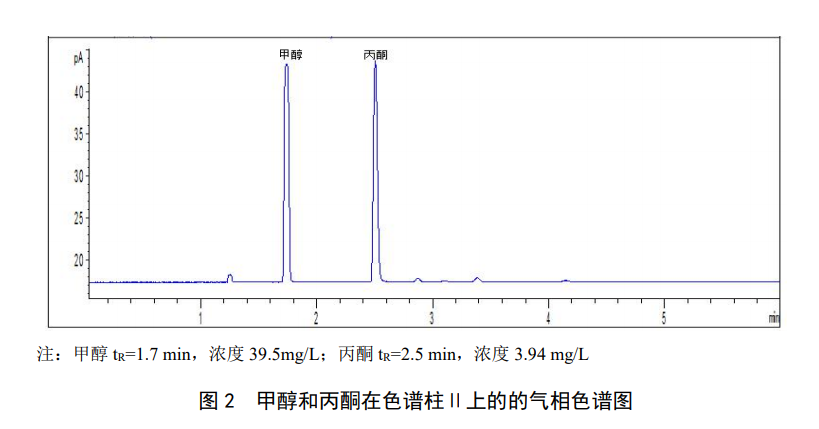
Quantitative results can be calculated and the concentration can be calculated according to the corresponding formula.
The above content comes from《HJ 895-2017 Water Quality-Determination of Methanol and Acetone-Headspace/Gas Chromatography》


