Sulfides in drinking water mainly come from the dissolution of underground minerals and the decomposition of microorganisms in water under anaerobic conditions. In addition to the above two types of sulfide in rivers, a part of it comes from domestic sewage and industrial wastewater. The detection methods mainly include iodometry and methylene blue spectrophotometry. The difference between the two methods is that the iodometry can only detect water samples with a sulfide content of 0.40 mg/L, while the methylene blue spectrophotometry has a lower limit of determination. At 0.04mg/L. Therefore, for some experiments that need to obtain accurate sulfide content in water, most of them will use methylene blue spectrophotometry.

After the sulfide in the water sample is acidified, heated with nitrogen, or distilled, the hydrogen sulfide generated is absorbed by sodium hydroxide solution, and the generated sulfide ion reacts with N,N dimethyl p-phenylenediamine in the acidic solution of ferric ammonium sulfate Methylene blue is generated, and its absorbance is measured at a wavelength of 665nm. The sulfide content is directly proportional to the absorbance value.
Unless otherwise specified, analytical reagents conforming to national standards are used for analysis, and the experimental water is deionized and deoxygenated water.
1. Deionized water
Pass distilled water through an ion exchange column to prepare deionized water, and pass nitrogen to saturation (at a rate of 200ml/min-300ml/min for approximately 20min) to remove dissolved oxygen in the water. The prepared deionized and deoxygenated water should be tightly covered immediately and stored in a glass bottle, ready to use. Or use freshly prepared ultrapure water from a pure water machine.
2. Sulfuric acid (H2SO4): p=1.84g/ml.
3. Hydrochloric acid (HCl): p=1.19g/ml, excellent grade pure.
4. Sodium hydroxide solution
Weigh 10.0g of sodium hydroxide and dissolve it in 1000ml of deionized water or ultrapure water, and shake it evenly.
5.N,N dimethyl p-phenylenediamine solution
Weigh 2.0g of N,N-dimethyl-p-phenylenediamine hydrochloride and dissolve it in 700ml of deionized water or ultrapure water, slowly add 200ml of sulfuric acid, dilute to 1000ml with water after cooling, and shake well. This solution is stored in a closed brown bottle at room temperature and is stable for three months.
6. Ferric Ammonium Sulfate Solution
Weigh 25.0g ferric ammonium sulfate and dissolve in 100ml deionized water or ultrapure water, slowly add 5.0ml sulfuric acid, dilute with water to 250ml after cooling and shake well. If insoluble matter appears in the solution, it should be filtered before use.
7. Zinc acetate.
8. Antioxidant solution
Weigh 4.0g ascorbic acid, 0.2g disodium edetate, and 0.6g sodium hydroxide, dissolve in 100ml deionized water or ultrapure water, shake well and store in a brown reagent bottle. Available now.
9. Disodium ethylenediaminetetraacetic acid.
10. Hydrochloric acid solution (1+1).
11. Acetic acid solution
12. Sulfide standard solution
This solution can be purchased directly in the relevant market, but you must purchase products with relevant qualifications.
13. Standard use fluid of sulfide
Transfer a certain amount of sulfide standard solution into a brown volumetric flask that has been added with 2.0ml sodium hydroxide solution and 100ml ultrapure water, and use deionized water or ultrapure water to make a constant volume to prepare a sulfide ion concentration of 10.00mg/ L's sulfide standard use fluid. Available now.
14. Nitrogen: purity ≥99.999%.
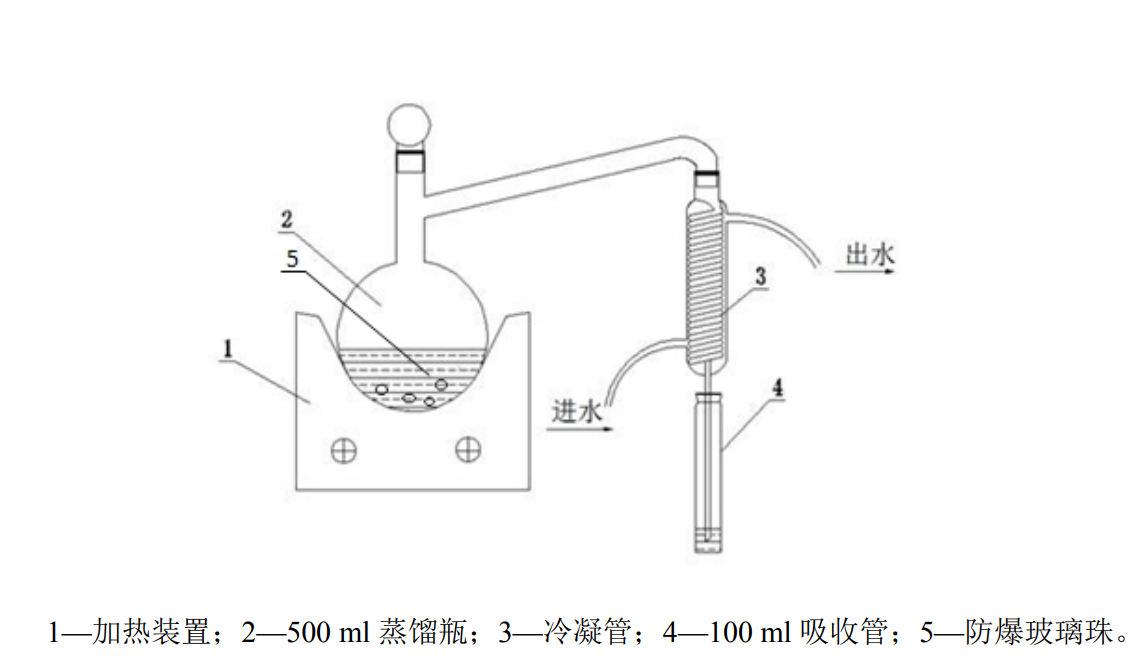
15. Acidification-blowing-absorption or acidification-distillation-absorption device
16. Spectrophotometer
17. A colorimetric tube with stopper 100ml.
18. Brown glass bottle with stopper and ground mouth 200ml.

There are two methods for sample preparation, one is the acidification-blowing-absorption method, and the other is the acidification-distillation-absorption method. We recommend that you use the distillation absorption method for these two methods. Because the nitrogen used in the acid blowing method contains a small amount of oxygen, it will oxidize the hydrogen sulfide in the water sample during the experiment, which will affect some low-concentration water samples (groundwater, drinking water).
Acidification-distillation-absorption method
Take 200ml of the mixed water sample, or dilute an appropriate amount of water sample with water to 200ml, quickly transfer to a 500ml distillation flask, then add 5ml of antioxidant solution, gently shake, and add a few glass beads. Measure 20.0ml sodium hydroxide solution in a 100ml absorption colorimetric tube as the absorption liquid, and insert the lower end of the distillate tube into the liquid surface of the absorption liquid to ensure complete absorption. Open the condensed water, quickly add 10ml of hydrochloric acid solution to the distillation flask, and immediately close the stopper, turn on the temperature-controlled electric furnace, adjust the appropriate heating temperature, and distill at a distillation rate of 2ml/min-4ml/min. When the solution in the colorimetric tube reaches about 60ml, stop the distillation. Disconnect the air duct, flush the distillate tube with a small amount of water, and merge it into the absorption liquid for testing.
1. Establish a standard curve
Take 6 100ml colorimetric tubes with stopper, add 20ml sodium hydroxide absorption solution to each, respectively measure 0.00ml, 0.50ml, 1.00ml, 2.00ml, 4.00ml, 7.00ml sulfide standard use solution into each colorimetric tube, Add water to about 60ml, slowly add 10ml N,N-dimethyl-p-phenylenediamine solution along the wall of the colorimetric tube, immediately plug tightly and slowly invert once, unplug the small mouth and add 1ml ferric ammonium sulfate solution, immediately plug tightly and fully Shake well and prepare a standard sequence with concentrations of 0.00ug/L, 5.0ug/L, 10.0ug/L, 20.0ug/L, 40.0ug/L, 70.0ug/L. After leaving it for 10 minutes, use deionized water or super Dilute the volume of pure water to the marking line and shake well. Use a 10mm cuvette with deionized water or ultrapure water as a reference, and measure the absorbance at a wavelength of 665nm. Take the sulfide content (ug) as the abscissa and the absorbance after deducting the zero concentration point as the ordinate to draw a standard curve for the determination of high-concentration samples.
Measure out 0.00ml, 0.20ml, 0.50ml, 1.00ml, 1.50ml, 2.00ml sulfide standard use solution respectively, according to the above steps, formulate the concentration to 0.00ug/L, 2.0ug/L, 5.0ug/L, 10.0 The standard sequence of ug/L, 15.0ug/L, and 20.0ug/L is colorimetric with a 30mm cuvette to draw a standard curve for the determination of low-concentration samples.
2. Water sample analysis
Operate the acidified and distilled water sample according to the above standard curve establishment steps, and then measure its absorbance, and compare the parameters of the blank sample measured in the same step to obtain the accurate content of sulfide in the water sample.
1. General sulfide water samples should be tested immediately after collection. If conditions do not permit, you can first add zinc acetate solution, then add the water sample to a nearly full bottle, then add sodium hydroxide solution, and finally add antioxidant solution. The collected water sample is fixed, but the measurement should be performed within 4 days.
2. Because some of the reagents used are dangerous, the waste generated during the experiment should be stored after proper treatment, and the corresponding identification should be made, and the qualified unit should be entrusted to deal with it to prevent harm to personnel and the environment .
3. Be careful when adding N,N-dimethyl-p-phenylenediamine and ferric ammonium sulfate, because both reagents contain corrosive liquids such as sulfuric acid. When adding these two reagents, they should be added slowly along the wall of the colorimetric tube, and then quickly plugged and mixed to avoid the loss of hydrogen sulfide escape.
4. Don't shake the water sample too much during collection to avoid the volatilization of sulfide.
References in this article: Water quality determination of sulfide-methylene blue spectrophotometric standard.
最新动态
相关推荐